- Call +91.22.23881724/23893165
- Mail director[dot]fwtrc[at]nic[dot]in
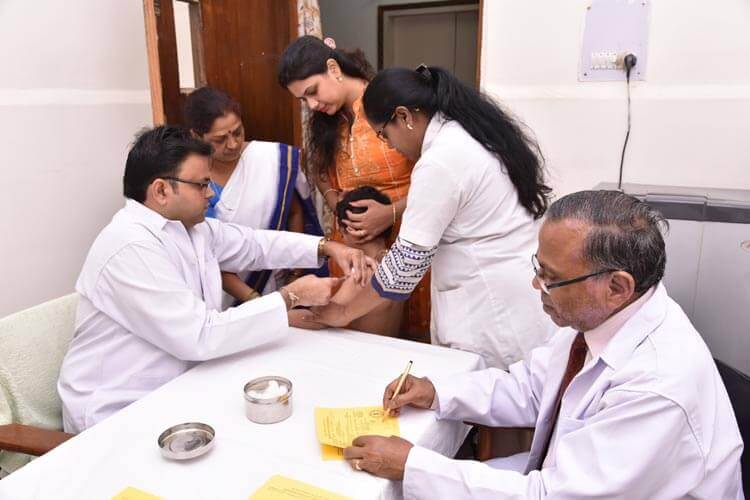
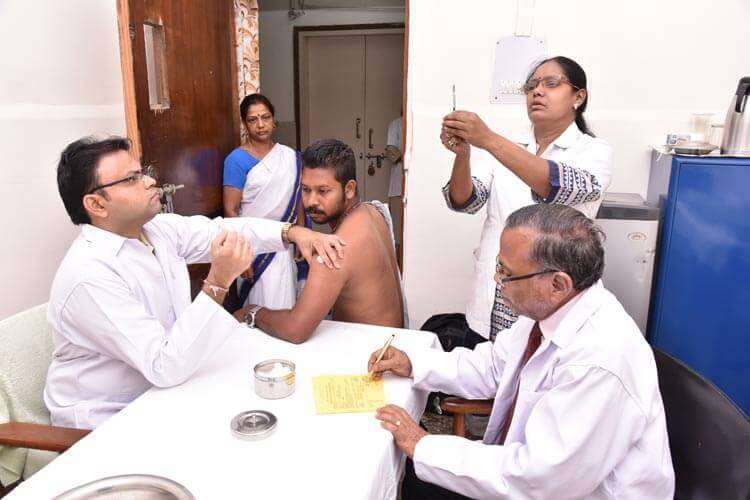
Yellow Fever Vaccination Centre
- Home
- Facilities
- Yellow Fever Vaccination Center
Yellow Fever Vaccination
| Title | Detail |
|---|---|
| Days : | Khetwadi campus: Monday Panvel campus: Tuesday |
| Timings Of Vaccination : | 2-4 pm |
| Pre-registration Form : | Desirable (Registration Form needs to be filled on website) |
| Cost of the Vaccine : | Rs 300/per dose |
| Mandatory Requirement : | Original passport (Individuals above the age of 60 yrs are also required to produce a medical certificate of fitness for Yellow Fever Vaccination) |
Yellow Fever Vaccine is an attenuated, live-virus preparation of the 17D strain of yellow fever virus grown in leucosis-free chick embryos. A single dose correctly given confers immunity in basically 100% of recipients. Protective immunity is achieved only after 10 days of yellow fever vaccination and persists for at least 10 years. This vaccine is given as a single injection given subcutaneously. Yellow fever Vaccination Certificate becomes valid only after 10 days of vaccination.
2.1 Vaccine summary
| Title | Summary |
|---|---|
| Type of vaccine | Live viral |
| Number of doses | One dose of 0.5 ml subcutaneously |
| Route of Administration | Sub-cutaneous |
| Schedule | Can be given at nine months of age |
| Booster | Not required (as per GOI order No L-21021/52/2013-PH(IH) dated 30/08/2017) |
| Contraindications | Egg allergy; immune deficiency from medication or disease; symptomatic HIV infection; hypersensitivity to previous dose; pregnancy |
| Adverse reactions | Hypersensitivity to egg; rarely, encephalitis in the very young; hepatic failure. Rare reports of death from massive organ failure. |
| Special precautions | Do not give before six months of age; avoid during pregnancy |
| Storage temperature | +2 to +8 degrees centigrade |
Source: WHO
2.2 Side Effects
Severe or serious adverse reactions to 17D vaccine are extremely rare. Post-vaccinal encephalitis (due to invasion of the brain by the vaccine virus) has long been recognized as a rare complication related to use of the vaccine in very young infants. 18 of the 21 reported encephalitis cases were in children, of whom 16 were under 7 months. Virus recovered from the brain of the single reported fatal case contained two amino acid changes in the E gene and exhibited increased neuro-virulence in animals. It is unknown whether the other cases were due to mutations in the vaccine virus. Anaphylactic reactions to yellow fever vaccine occur at a frequency of approximately 1/58000 and may be due to sensitivity to gelatin used to stabilise the vaccine.
1. Mild Side Effects of Vaccination
- Yellow fever vaccine has been associated with fever and with aches, soreness, redness or swelling where the shot was given. These problems occur in up to 1 person out of 4. They usually begin soon after the shot, and can last up to a week.
- Most people will get a slight sore arm
- 2-10% may feel tired, headache, muscle aches, fever for 24 hours starting 3-9 days after the vaccine
- 1% need to curtail regular activities
2. More Serious Side Effects of Vaccination
- The risk of a vaccine causing serious harm, or death, is extremely low.
- Severe allergic reaction to a vaccine component (about 1 person in 58,000).
- Severe nervous system reaction (about 1 person in 125,000).
- Life-threatening severe illness with organ failure (about 1 person in 250,000). More than half the people who suffer this side effect die. These last two problems have never been reported after a booster dose.
- 1 in 130,000 will get immediate hypersensitivity – rash, itching faint or asthma – this is why you need to wait 30 minutes in the clinic
- 0.09-2.5 per million will get inflammation of multiple organs e.g. lungs, kidney, liver, spleen, skin, blood stream
- 1 in 8 million will get encephalitis (Inflammation of the brain)
Nodal Officer
Dr. Suparna Khera, Chief Medical Officer (SAG)
National Institute of Public Health Training and Research,
332, S.V.P. Road, Khetwadi, Mumbai - 400 004, Maharashtra, India
Phone :
022-23881724 / 23893165